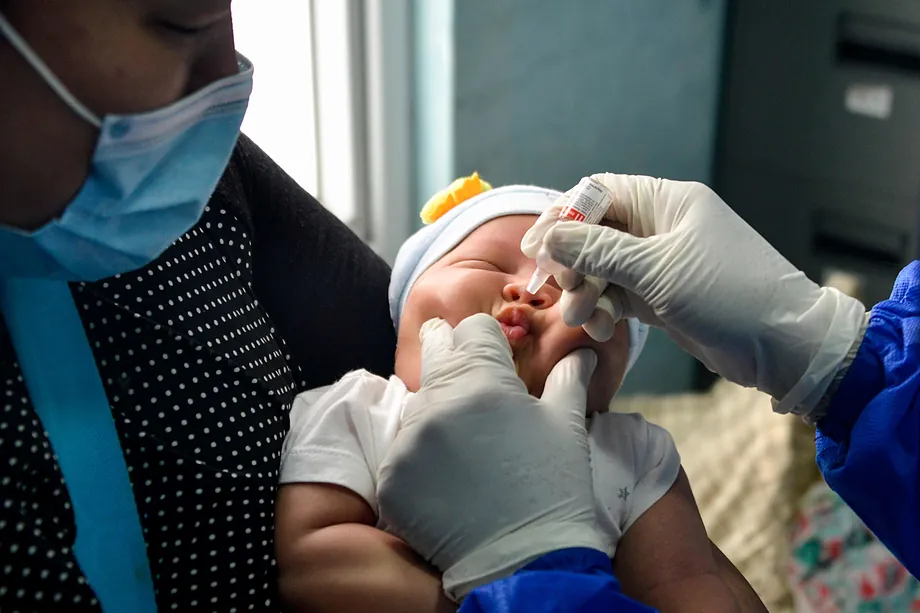
Under the EU4Health program, HaDEA’s support will accelerate the final stages of clinical development of the MTBVAC vaccine candidate by facilitating the completion of a Phase 3 clinical trial in newborns, which is currently underway in different countries on the African continent. The objective of the clinical trial is to evaluate the protective efficacy, safety and immunogenicity of a single dose of MTBVAC administered intradermally to healthy unexposed newborns (HUU) and non-exposed newborns (HEU).
Advertising
A safe, effective, equitable and affordable TB vaccine such as MTBVAC will be an important tool to stop the development and spread of drug-resistant TB, which is a major concern for health systems. The same will happen in Europe, where an annualized increase in the incidence of multidrug-resistant tuberculosis (MDR/XDR-TB) has been recorded for the first time in a decade. An increase that may be a consequence of the interruption of infection control programs as a result of the COVID-19 pandemic.